
Advancing Healthcare Through Clinical Research
Partner with a leading clinical research site conducting Phase I-IV trials across multiple therapeutic areas. Join us in shaping the future of medicine.

Partner with a leading clinical research site conducting Phase I-IV trials across multiple therapeutic areas. Join us in shaping the future of medicine.
A well-established site focused on the quest for excellence in medical research and healthcare delivery
At OSIS Clinical Research, we are dedicated to advancing medical science through rigorous, ethical clinical trials. Our experienced team of principal investigators, research coordinators, and healthcare professionals work tirelessly to bring new treatments and therapies to patients who need them most.
With extensive experience conducting Phase I-IV Clinical Trials, we bridge the gap between innovative pharmaceutical research and real-world patient care. Our commitment to excellence ensures the highest quality data and participant safety in every study we conduct.

Recognized for excellence in clinical trial management
Full-service clinical trial support from protocol development to study completion
Comprehensive support for all clinical trial phases, from first-in-human studies to post-marketing surveillance.
Access to a diverse patient population with efficient recruitment strategies and strong community relationships.
Strict adherence to FDA regulations, ICH-GCP guidelines, and institutional review board requirements.
On-site laboratory capabilities with certified specimen processing and logistics coordination.
Electronic data capture systems with real-time query resolution and data quality monitoring.
Comprehensive patient support services ensuring participant safety and study retention.
Extensive experience across multiple disease states and patient populations
Hypertension, Hyperlipidemia, Heart Failure
Diabetes Mellitus, Obesity, Liver Cirrhosis
Asthma, COPD, Respiratory Infections
Rheumatoid Arthritis, Gout, Osteoarthritis
Alzheimer's, Parkinson's, Multiple Sclerosis, Migraine
Anxiety, Depression, Bipolar Disorder, ADHD
Insomnia, Sleep Disorders
Psoriasis, Eczema, Rosacea, Atopic Dermatitis
Hormonal Studies, Reproductive Health
Lupus, HIV
Preventive Vaccines, Immunization Studies
Phase I Studies, PK/PD Studies
Your trusted partner for successful clinical trial execution

Access to a large, diverse patient database ensures quick recruitment timelines and study completion.
Rigorous quality control processes deliver clean, audit-ready data with minimal queries.
Our principal investigators and coordinators have decades of combined clinical trial experience.
State-of-the-art research facility equipped for all study requirements and patient comfort.
Make a difference in healthcare while receiving cutting-edge medical care
Be among the first to receive potentially life-changing therapies before they're available to the public.
Receive comprehensive healthcare from experienced physicians and research professionals at no cost.
Many studies offer compensation for your time and travel expenses.
Help researchers develop treatments that could benefit millions of people worldwide.
Evaluating a new oral medication for blood sugar control
Non-opioid treatment for chronic lower back pain
New combination therapy for blood pressure management
Healthy volunteer vaccine immunogenicity study
Investigating new treatments for systemic lupus erythematosus
Clinical trial for liver cancer treatment options
Evaluating new therapeutic approaches for major depressive disorder
New treatment options for chronic eczema and skin inflammation
A clinical trial is a research study that tests new medical treatments, drugs, or devices to see if they are safe and effective. By participating, you help advance medical knowledge while potentially accessing new treatments.
Patient safety is our top priority. All studies are reviewed by ethics committees (IRBs), follow strict FDA guidelines, and you'll be closely monitored throughout. You can withdraw at any time.
Many studies offer compensation for your time and travel expenses. The amount varies by study. All study-related medical care, tests, and medications are provided at no cost to you.
Each study has specific criteria. Contact us to discuss your health history and we'll help identify studies you may qualify for. There's no obligation to participate after the initial screening.
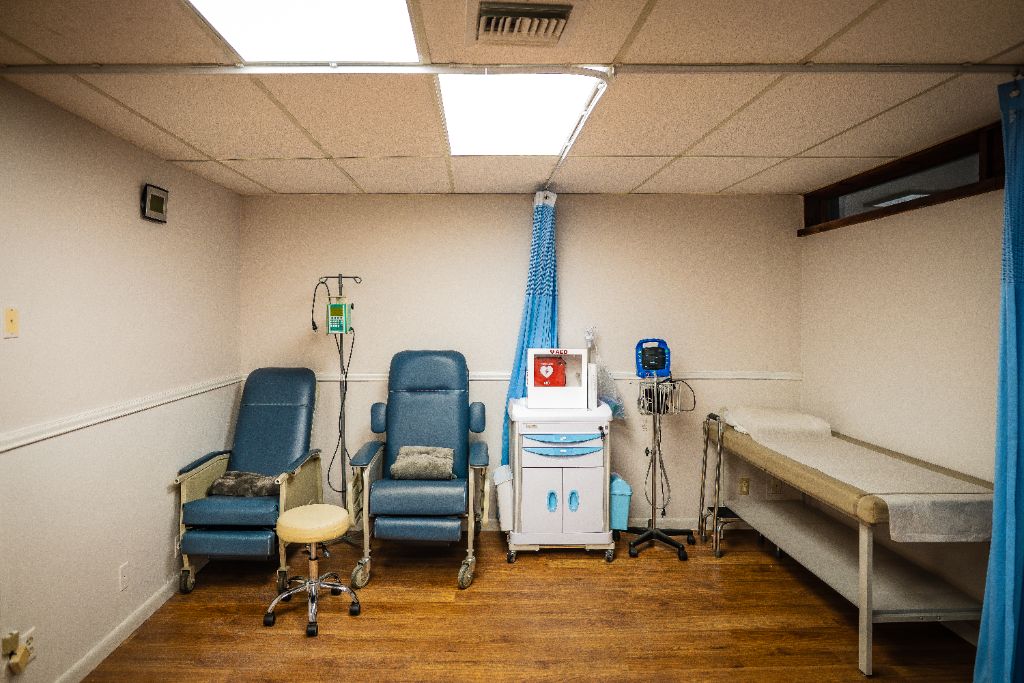
Explore our state-of-the-art clinical research facility











Select a date and time that works best for you
Please select a date to see available times
"Partnering with OSIS for our Phase III diabetes trial was an excellent decision. Their enrollment exceeded targets by 40% and data quality was exceptional."
"Participating in the clinical trial at OSIS changed my life. The staff was caring and professional, and I received treatments that significantly improved my condition."
"The OSIS team's attention to protocol compliance and patient safety is unmatched. They're our go-to site for complex therapeutic studies."
"I was nervous about joining a clinical trial, but the OSIS team made me feel safe and informed every step of the way. They truly care about their patients."
"We've worked with dozens of research sites globally, and OSIS consistently delivers top-tier results. Their diverse patient population and rapid enrollment capabilities are unmatched."
We're here to answer your questions and discuss how we can work together
540 E. McNab Rd
Suite B
Pompano Beach, FL 33060
Main: (954) 743-1121
Fax: (954) 674-2087
info@osisclinicalresearch.com
sponsors@osisclinicalresearch.com
patients@osisclinicalresearch.com
Monday - Friday: 9:00 AM - 5:00 PM
Saturday: Closed
Sunday: Closed